Frontage Holdings Announces 2022 Annual Results
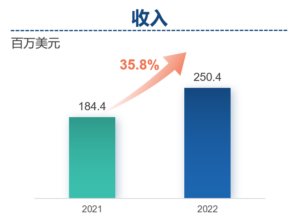
Revenue increased by 35.8%year-on-year to US$250.4 million
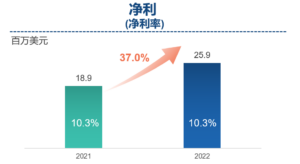
Net profit increased by 37.0% year-on-year to US$25.9 million
Basic and diluted earnings per share increased by 40.0%and 41.4% year-on-year to US$0.0126 and US$0.0123
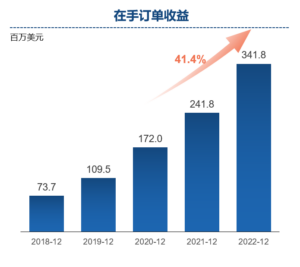
Contract future revenue increased by 41.4% year-on-year to US$341.8 million
Hong Kong, March 28, 2023 – Frontage Holdings Corporation (“Frontage” or “Frontage Holdings”, stock code: 1521.HK), a contract research organization (“CRO”) providing integrated, science-driven research, analytical and development services with presence in both North America and China, today announces its audited annual results for the year ended December 31, 2022.
Financial Highlights
- Revenue of Frontage increased by 35.8% year-on-year to approximate US$250.4 million, despite the adverse effects of COVID-19 in China.

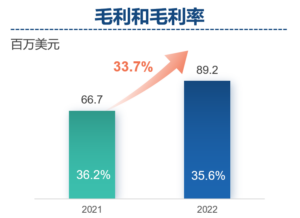
- Gross profit of Frontage increased by 33.7% year-on-year to US$89.2 million. Gross profit margin of Frontage slightly decreased from 36.2% in 2021 to 6% in 2022, primary due to the adverse effect of COVID-19 and lower margin contributed by newly established services in new facilities opened in 2022.

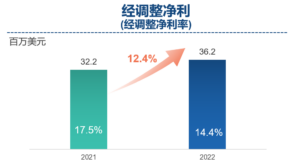
- Net profit increased by 37.0% year-on-year to US$9 million, excluding unusual, non-recurring, non-cash and/or non-operating items. Adjusted Non-IFRS net profit increased by 12.4% year-on-year to US$36.2 million. Net profit margin was 10.3%, same as 2021, while adjusted net profit margin was 14.4% compared to 17.5% in 2021, primarily due to the adverse effects of COVID-19 and lower margin contributed by newly established services in new facilities.


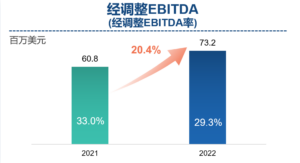
- EBITDA increased by 35.5% year-on-year to US$9 million, excluding unusual, non-recurring, non-cash and /or non-operating items. Adjusted Non-IFRS EBITDA increased by 20.4% to US$73.2 million.

- Basic and diluted earnings per share increased by 40.0% and 42.5% year-on-year to US$0.0126 and US$0.0124, respectively.
Operational Highlights
- Contract future revenue reached US$8 million as of December 31, 2022, reflecting a 41.4% increase from US$241.8 million as of December 31, 2021.

- Further expansion of services offerings and increased integration of our drug discovery and development platform in North America:
- Expanded capacities and capabilities in pharmacological safety assessment and toxicology services through the acquisition in January 2022 of Experimur LLC, a CRO located in Chicago, U.S., which provides full GLP-compliant toxicology and featuring developmental reproductive, carcinogenicity, ocular and general toxicity studies.

- Expanded our DMPK portfolio to include enhanced comprehensive scientific expertise in metabolite identification, QWBA, non-GLP bioanalysis and IND-enabling studies, created a center of excellence in metabolite identification/profiling by integrating the operations of RMI Laboratories, LLC into our DMPK units in Exton, and strengthen drug transporter research.
- Grew our central laboratory unit in Exton, PA by expanding logistic service for kits and sample management solutions, biobanking services histology pathology testing services, and clinical trial safety tests to support a higher volume of clinical trials.

- Opened a new state-of-the-art 25,000 sq. ft. facility in Hayward, CA in May 2022, which further expands our bioanalytical, biologics bioassay, and biomarker services in North America. We have also expanded our business in biologic drug products testing by constructing a new microbiology lab in the CMC unit.

- Expanded early-phase clinical research studies services through the acquisition of Frontage Clinical Services Inc., a CRO located in Secaucus, NJ., principally engaged in provision of CRO services relating to early phase clinical research studies.

- Completed the design for a new manufacturing facility spanning 46,000 square feet in Exton, PA, featuring nine manufacturing suites, formulation labs, and analytical testing labs. The construction of this cutting-edge facility is projected to be finalized by the end of the first quarter of 2024. This expansion will significantly elevate our manufacturing capacity, allowing us to produce batches for late-phase clinical trials.
- In China, most of our new service facilities commenced operations in 2022, enabling us to provide integrated services encompassing early drug discovery, preclinical research, development and production of API and formulations. Revenue in China from innovative drugs development projects contributed over 70% of the total in 2022, contract future revenue from innovative drugs development projects contributed over 80% of the total as of December 31, 2022, compared with approximately 65% and 50% as at December 31, 2021 and December 31, 2020, respectively.
- Expanded our services offerings to strengthen integrated drug discovery and development platform in China:
- The 215,000 sq. ft. preclinical animal research facility in Suzhou has been operational since January 2022.The facility successfully completed the on-site inspection by AAALAC international certification experts at the end of September 2022 and obtained AAALAC certification in March 2023.

default - The 67,000 sq. ft. research facility in Lin-Gang, Shanghai has been partially operational since the third quarter of 2022, in which we began to provide DMPK in vitro research and large molecular bioanalysis services, significantly enhancing our original DMPK and bioanalytical and biologicals, biomarker capacity.

- The 34,000 sq. ft. pharmacodynamic research facility in Wuhan has been operational since June 2022, enabling us to expand our enzymatic platform and cell line construction platforms and establish a electrophysiological platform. We also established an in vivo drug efficacy evaluation service in our preclinical animal research facility in Suzhou, expanding our drug efficacy research services from in vitro drug efficacy evaluation to in vivo drug efficacy evaluation.

- A 7,000 sq. ft. GMP kilo laboratory in Acme Shanghai site became fully operational in the first half of 2022, enabling us to offer non-GLP/GLP/GMP batch production to our customers. Additionally, we expect our synthetic and medicinal chemistry facility in Wuhan, covering an area of 200,000 square feet, to become partially operational by the first half of 2023.

- Continued to strengthen capabilities in bioanalytical and biologicals, specifically in the fields of antibody drug conjugates, liposome compounds, and endogenous compound analysis. We also developed new platforms in oligonucleotides, cell and gene therapy, protein/peptide, and insulin bioanalysis. A GLP bioanalytical laboratory was set up within our preclinical business unit in Suzhou to support TK sample analysis. In addition, the central laboratory unit in Shanghai initiated service offerings for electronic sample management services and laboratory testing.
- Completed the construction of a new 89,000 sq. ft. clinical sample manufacturing facility in Suzhou and began trial operations in January 2023, which will strengthen our competency in clinical trial sample/material production in various dosage forms, including injections, semi-solid preparations, and eye drops.

- Chenghong Pharmaceutical (Weihai) Co., Ltd., an associate of Frontage, completed its phase I construction of a cGMP API manufacturing facility on a 11-acre land in Weihai, China. The facility includes a total of 120,000 sq. ft facility and a total of 50,000 L of reactor volume with high pressure, low temperature, and hydrogenation chemical process capabilities.

- With the opening of new facilities and expansion of capacity and capabilities of each service platform, our headcount increased to 1,698 as of December 31, 2022 from 1,322 as ofDecember 31, 2021. Headcount located in North America and China grew to 732 and 966, respectively, as of December 31, 2022, up from 567 and 755, respectively, as of December 31, 2021.
Management Comments
Dr. Song Li, Founder and Chairman of Frontage Holdings, commented: “As in previous years, we are proud of Frontage’s significant growth and milestones achieved in 2022. Despite the challenges presented by the ongoing COVID-19 pandemic lockdowns in China, inflation, and labor shortages in North America, our resilience and strength enabled us to continue investing in our employees, operational excellence, organic expansion, mergers and acquisitions, and long-term development objectives. Our revenue increased by 35.8% year-over-year, and net profit increased by 37.0% year-over-year. We also achieved a record-high contract future revenue of US$341.8 million as at December 31, 2022, representing a 41.4% increase over the past 12 months.
Despite the physical lockdown and shutdown of our facilities in Shanghai and Zhengzhou and the high number of COVID-19 cases among employees, our teams in China demonstrated resilience and employed various mitigation measures to minimize adverse impacts on our ongoing projects, customer relationships, and procurement of supplies and materials. Following the lifting of China’s COVID-19 containment measures and the sharp drop in the number of COVID-19 cases across the country, all of our businesses in China resumed normal operations in early 2023.
Frontage’s commitment to building and developing an extensive drug discovery and development service platform and offering comprehensive one-stop services to clients globally has never wavered. In 2022, with the commencement of operation of our new facilities in China, including Suzhou preclinical animal research facility, Shanghai Lin-Gang laboratory and Wuhan pharmacodynamics laboratory, we built early-stage discovery and pre-clinical service capability, include drug efficacy research, DMPK, safety and toxicology, and other related services. These new facilities are still in the capacity ramping-up period and our focus in 2022 on establishing high quality systems and operating procedures resulted in a comparatively lower gross profit margin.
Revenue from these new service facilities has reached 14% of the company’s revenue in China within the first year of operation.
As a full-service CRO operating in the dynamic and constantly evolving life sciences industry, Frontage recognizes the critical role that market trends play in shaping our business prospects. The life sciences industry is an essential component of the global healthcare system, and we are confident that it will continue to experience steady growth in the future. The increasing trend towards outsourcing drug discovery and development services to CROs is expected to persist, driven by the growing complexity of drug R&D, the need for specialized expertise, and the desire to reduce costs and increase efficiency.
Looking forward, we will continue to optimize our integrated service platform, ensuring that we deliver high-quality services that cover early drug discovery to drug development services. We will also expand our areas of expertise, offering cutting-edge and leading technology platforms to attract new clients and deepen our relationships with existing ones. We are dedicated to improving our unique internationalization strategy, which involves adhering to the same quality system standards between China and the United States. Leveraging our business layouts in both North America and China, we will share cutting-edge technology, project experience, quality systems, and other positive resources while operating independently in both areas. This approach will enable us to provide high-quality services to customers worldwide and position ourselves as the preferred partner for biopharmaceutical companies globally.
Forward-Looking Statements
This presentation may contain certain “forward-looking statements” which are not historical facts, but instead are predictions about future events based on our beliefs as well as assumptions made by and information currently available to our management. Although we believe that our predictions are reasonable, future events are inherently uncertain and our forward-looking statements may turn out to be incorrect. Our forward-looking statements are subject to risks relating to, among other things, the ability of our service offerings to compete effectively and our ability to meet timelines for the expansion of our service offerings. Our forward-looking statements in this presentation speak only as of the date on which they are made, and we assume no obligation to update any forward-looking statements except as required by applicable law or listing rules. Accordingly, you are strongly cautioned that reliance on any forward-looking statements involves known and unknown risks and uncertainties. All forward-looking statements contained herein are qualified by reference to the cautionary statements set forth in this section.
Use of Adjusted Financial Measures
We have provided adjusted net profit, adjusted net profit margin, adjusted basic and diluted earnings per share (excluding the share-based compensation expenses, amortization of acquired intangible assets from and expenses relation to mergers and acquisitions, gain arising from fair value change of previously held interest in an associate, and gain or loss arising from financial liabilities measured as fair value through profit or loss) as additional financial measures, which are not required by, or presented in accordance with, the IFRS. We believe that the adjusted financial measures used in this presentation are useful for understanding and assessing underlying business performance and operating trends, and we believe that management and investors may benefit from referring to these adjusted financial measures in assessing our financial performance by eliminating the impact of certain unusual, non-recurring, non-cash and/or non-operating items that we do not consider indicative of the performance of our business. However, the presentation of these non-IFRS financial measures is not intended to be considered in isolation or as a substitute for the financial information prepared and presented in accordance with the IFRS. You should not view adjusted results on a stand-alone basis or as a substitute for results under IFRS, or as being comparable to results reported or forecasted by other companies.